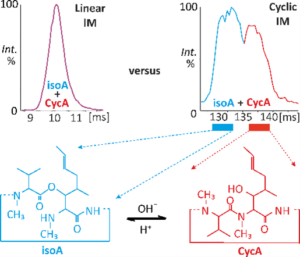
The Institute of Microbiology in Prague and Palacky University in Olomouc shared their instrumentation and reported in their paper that cyclic traveling wave ion mobility mass spectrometry (IM-MS) reveals an N → O peptidyl shift in singly protonated cyclosporin A (CsA), an important immunosuppressant used in transplantation surgeries, to isocyclosporin (isoA). This is a problem as the resulting isoA is an artifact of an analytical method that can artificially reduce the correct concentration of CsA, for example in pharmaceutical form.
No such isomerization was observed for doubly protonated and sodiated molecules. The article describes the use of sodium ion stabilization to facilitate the simultaneous separation and quantitation of singly charged cyclosporin isomers. The limit of detection and coefficient of determination were found to be 1.3% and 0.9908 for CycA in isoA and 1.0% and 0.9830 for isoA in CycA, respectively. These numerical parameters indicate the quality of the analytical determination.
A joint publication in Analytical Chemistry demonstrated the effectiveness of cyclic ion mobility spectrometry in separating CsA and isoA, which are cyclic undecapeptides with very similar collision cross sections. This is important because so far applications of ion mobility spectrometry have mostly been limited to substances that are very different in conformation.
The conformational flexibility of the molecule may pose a challenge for NMR spectroscopy experiments in protic solvents. However, our 1H-13C gHSQC NMR experiments allowed for the simultaneous recording of up to 11 cyclosporin conformers. The ratios were determined by integrating the volume of cross-peaks of the upfield resonating hydrogen in the diastereotopic methylene group of sarcosine-3. The finding is beneficial for the NMR field itself, where this application has reached the very edge of the method’s capabilities.